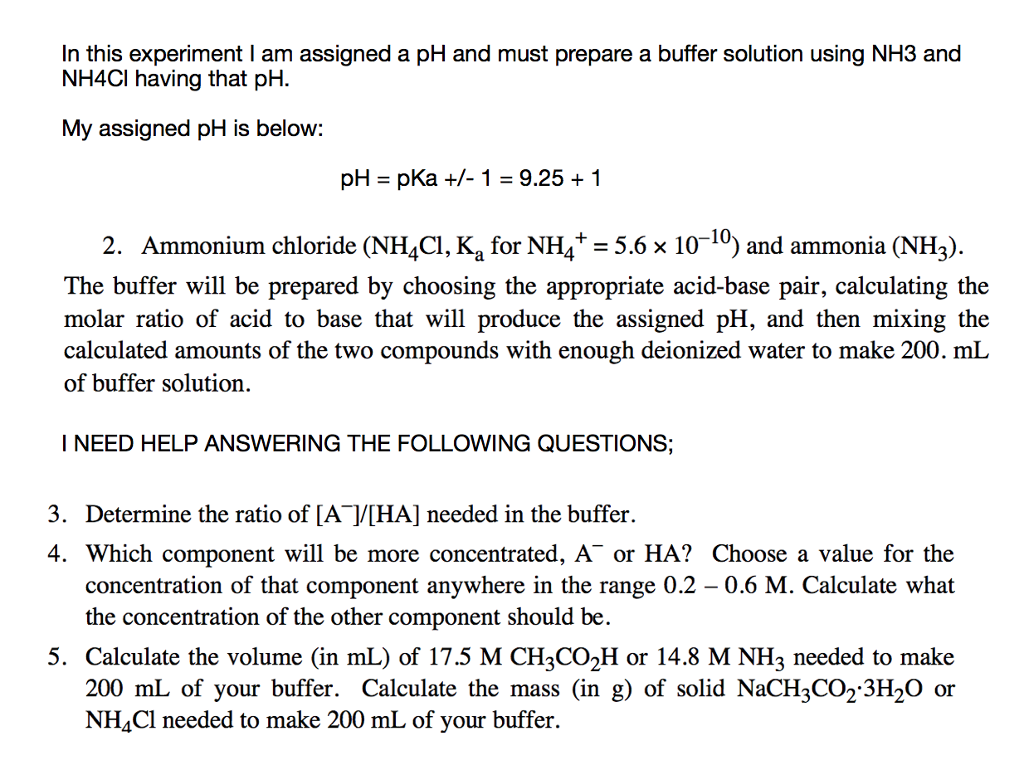
pH calculation of a buffer solution made from a weak base and its conjugate acid (salt form) - YouTube

OneClass: A)Calculate the pH of the 0.20 M NH3/0.24 M NH4Cl buffer. B)What is the pH of the buffer af...

Table 1 from VOLTAMMETRIC BEHAVIOURS OF Zn(II) AND Ni(II) COMPLEXES WITH ACID RED 1 AT MERCURY ELECTRODE | Semantic Scholar

OneClass: Calculate the pH of a .20M NH3/.20M NH4Cl buffer after the addition of 20.0mL of 0.10M HCl ...

A buffer solution is prepared by adding NH4Cl to a solution of NH3 (ammonia). NH3(aq) + H2O(l) = NH4+ (aq) + OH-(aq) What happens

ka = 7 x 10.) 31. An ammonia-ammonium chloride buffer has a pH value of 9 with (NH3) = 0.25. By how much the pH will change if 75 mL of 0.1
Calculate the amount of NH3 and NH4Cl required to prepare a buffer solution of pH = 9 when total concentration of buffering - Sarthaks eConnect | Largest Online Education Community

OneClass: does the addition of HCl to a NH4Cl/ NH3 buffer solution produce the common ion effect? why...








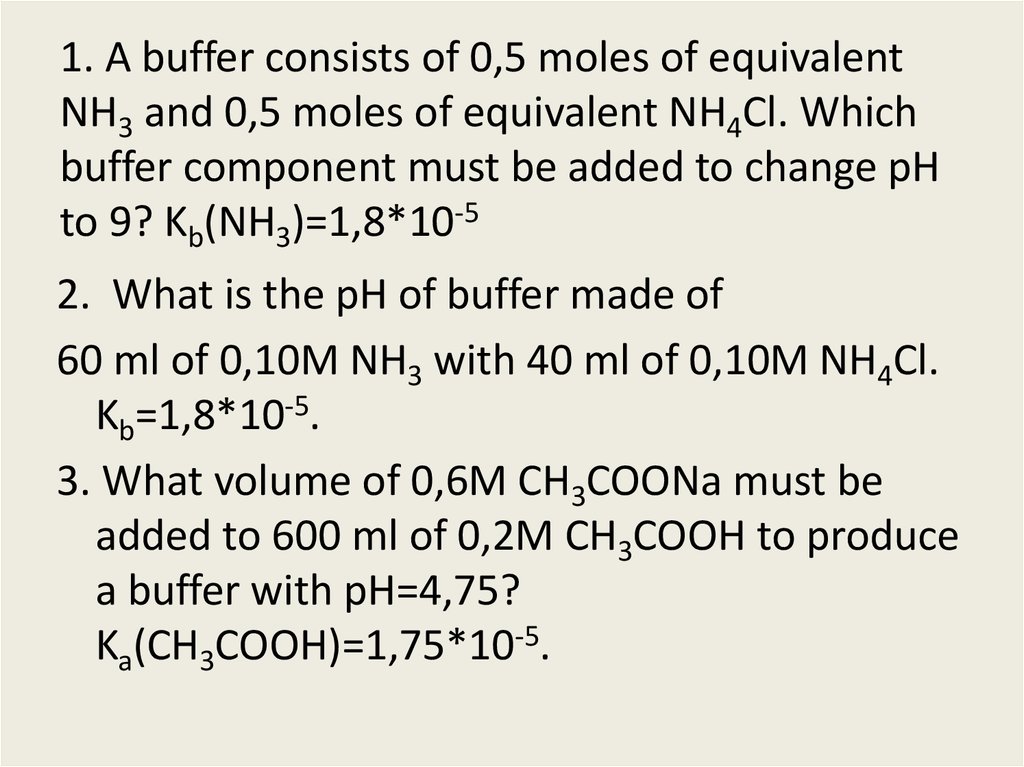


![Solved: BUFFERS 10. An Ammonium[NH3]/ammonium Chloride (NH... | Chegg.com Solved: BUFFERS 10. An Ammonium[NH3]/ammonium Chloride (NH... | Chegg.com](https://media.cheggcdn.com/media/98f/s907x655/98fc93a3-c6f6-4a89-8acb-45d7d9f5eb4d/image.png)